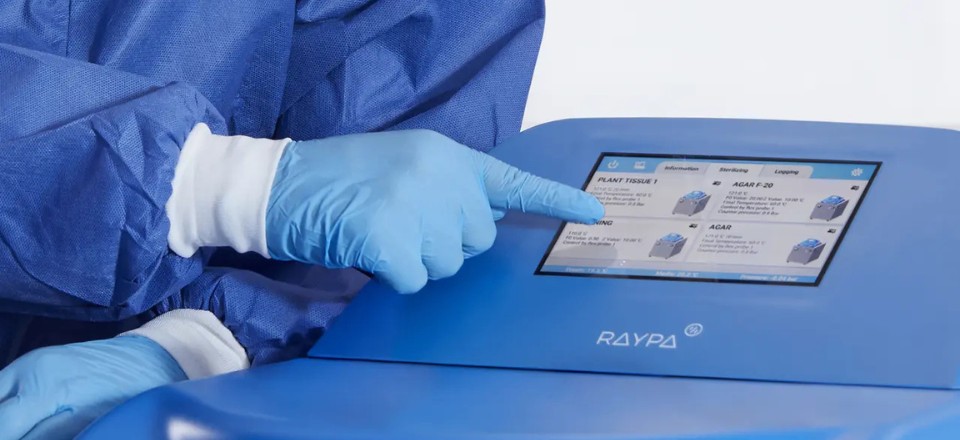
Digital Quality Management Options for Top Line Autoclaves
Digital quality management ensures traceability and efficiency in every cycle. Discover our six options, tailored to different levels of connectivity, privacy, and compliance.

In regulated environments, digital quality management is not optional—it is a strategic necessity. Traceability, regulatory compliance, and immediate access to information are essential to ensure safe, auditable, and efficient processes.
At RAYPA, we develop advanced digital quality management solutions tailored to the specific requirements of each laboratory. We offer 6 options to manage the quality of data stored both in the autoclave’s microprocessor and in the RAYPAcloud management platform, with different levels of connectivity, privacy, and FDA compliance.
Our Top Line autoclaves and media preparators are our flagship products and the preferred choice of pharmaceutical companies, biotechnology firms, and research centers in more than 100 countries. Designed with cutting-edge technology, they enable compliance with GMP and FDA regulations, operation in fully private environments, or seamless integration with LIMS and Active Directory systems.
Below, you will find all the available options, their features, and benefits—so you can choose the one that best fits your laboratory.
Most Popular Options
Private Standard
Maximum security and FDA compliance
Data is stored both in the autoclave controller and on a private server, with access to the offline management platform via a local area network. Both the controller and the platform are fully FDA 21 CFR Part 11 compliant. Includes automatic backups, complete audit trails, synchronous remote diagnostics via TeamViewer®, and manual updates.
✅ Ideal for industries that require maximum security, complete control, and certified traceability.

Cloud Standard
Full connectivity from anywhere
Stores data in both the controller and the cloud (AWS USA or EU), allowing online access to the platform from any device. Provides centralized management of multiple units, remote administration of users and programs, alert notifications, asynchronous remote diagnostics, and over-the-air updates.
✅ Perfect for those seeking flexibility, remote management, and high availability without the need for FDA compliance.

Other Options
Cloud-comply
Partial FDA compliance with advanced connectivity
Data is stored in both the autoclave controller and the cloud (AWS USA or EU), allowing online access to the management platform from any internet-connected device. Controller management complies with FDA 21 CFR Part 11, but cloud storage is not Part 11 compliant.
✅ Designed for users who require regulatory compliance on the equipment itself while also benefiting from advanced monitoring and remote administration features.
Essential-comply
FDA compliance without connectivity
All data is stored exclusively in the autoclave controller, configured to enable features such as audit trails, backups, and other tools that ensure compliance with FDA 21 CFR Part 11. It does not support remote access or cloud connection.
✅ Recommended for laboratories that do not require remote access but need to guarantee the integrity, traceability, and legal validity of their records.
Private Basic
Total privacy without connectivity
Data is stored on a private server, with access to the offline management platform through the local area network. While it is not FDA 21 CFR Part 11 compliant, it offers maximum internal security for those who prefer to keep all data within their own infrastructure without relying on cloud services.
✅ Ideal for environments with strict internal privacy policies.
Essential
Basic solution without connectivity
Stores data exclusively in the autoclave controller, with no cloud access or advanced digital management functions. It is not FDA 21 CFR Part 11 compliant.
✅ The simplest and most economical option for users who require direct, straightforward management.
Comparative Overview of the Options
We have prepared a summary document with the specifications of each option so you can easily compare levels of connectivity, privacy, and regulatory compliance, and choose the one most suitable for your laboratory.
Expert Support and Comprehensive Assistance
At RAYPA, we don’t just provide technology—we also ensure its successful implementation:
- Personalized consulting
- Custom developments and tailored integrations
- Equipment and software qualification
- Training on use and maintenance
- Advanced technical support and maintenance
Take the Next Step Towards Full Digital Traceability
Optimize your laboratory’s quality management, reduce audit times, and enhance the security of your processes.
Request an expert consultation or a demo today and discover which option is best suited for your laboratory.