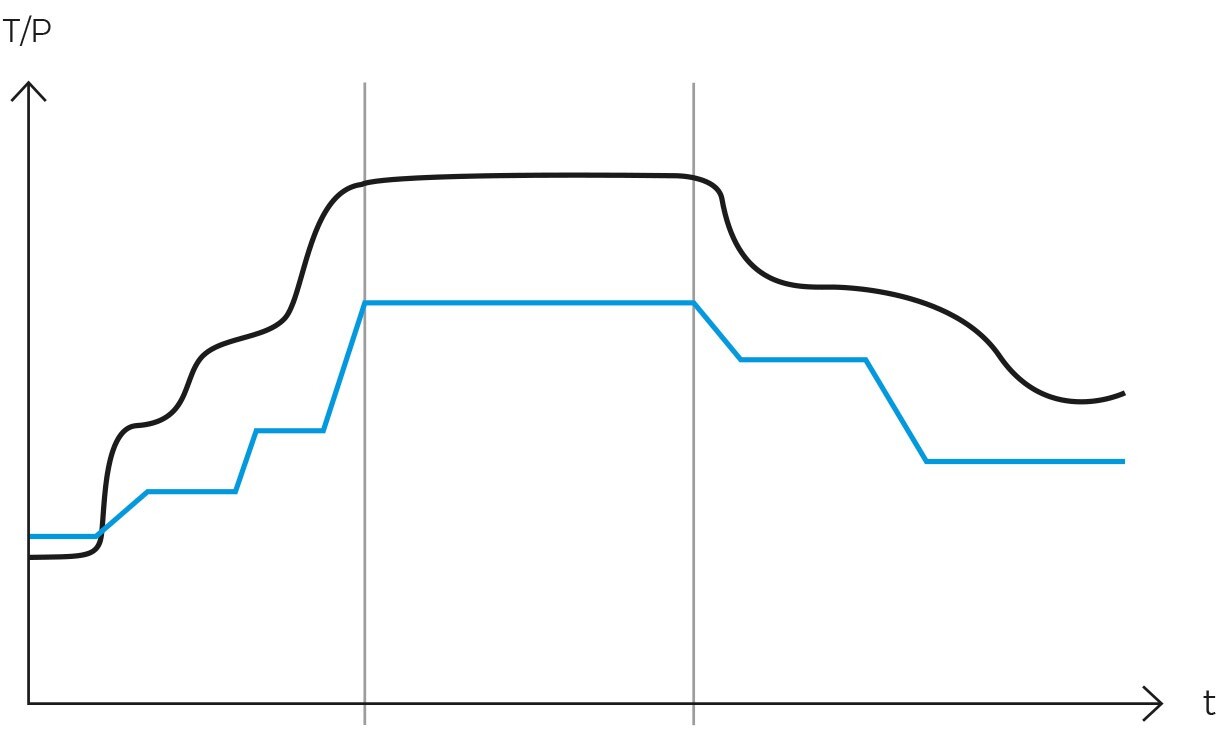
The gravity cycle has long been recognized as a traditional and extensively utilized methodology for wet sterilization via autoclaving. Its inherent simplicity, efficacy, and cost-effectiveness render it an appealing option across diverse settings.
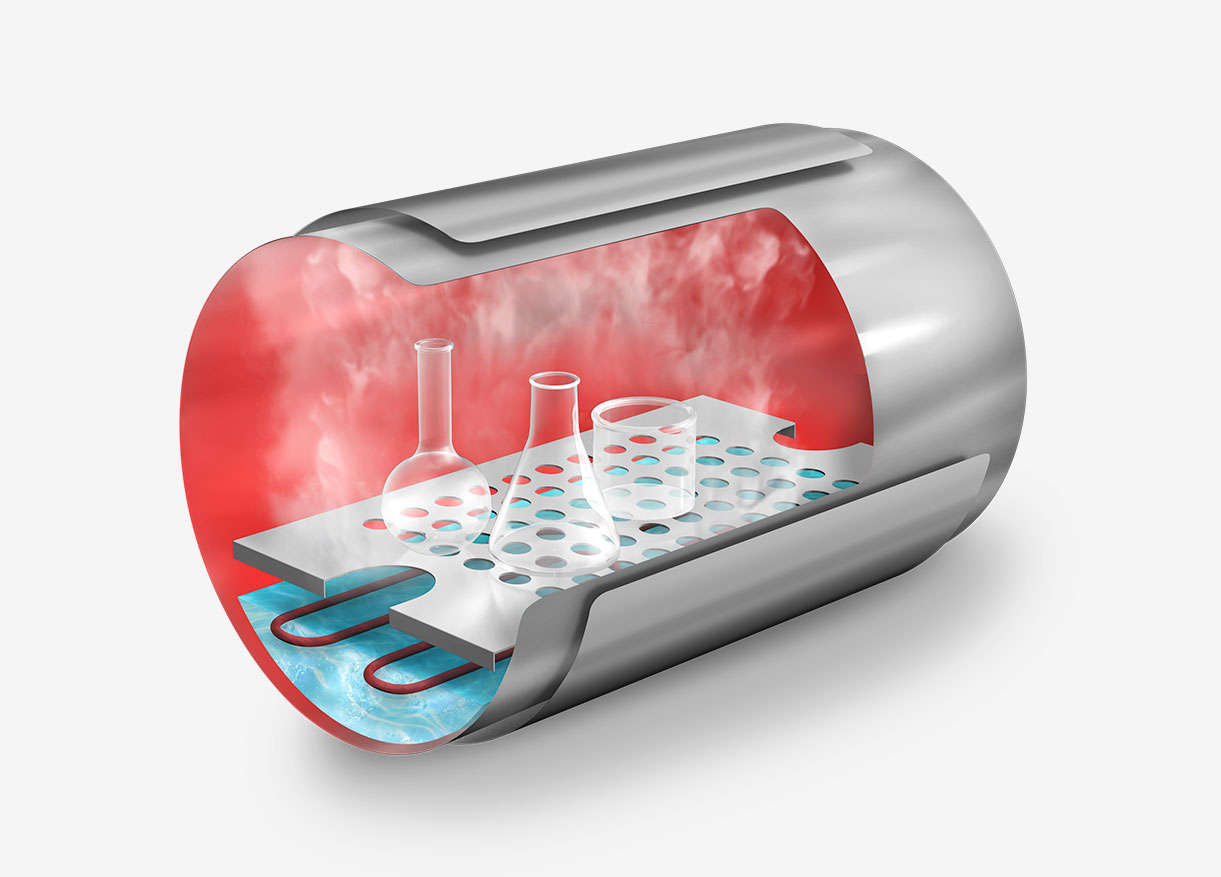
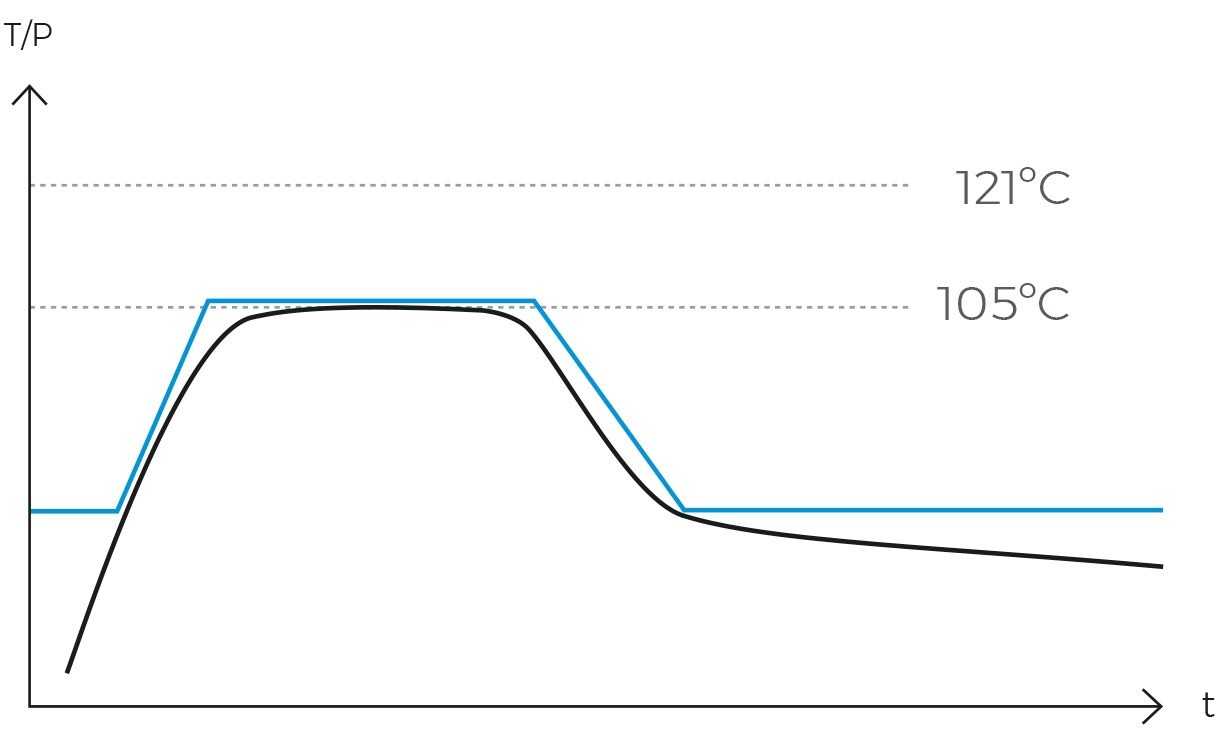
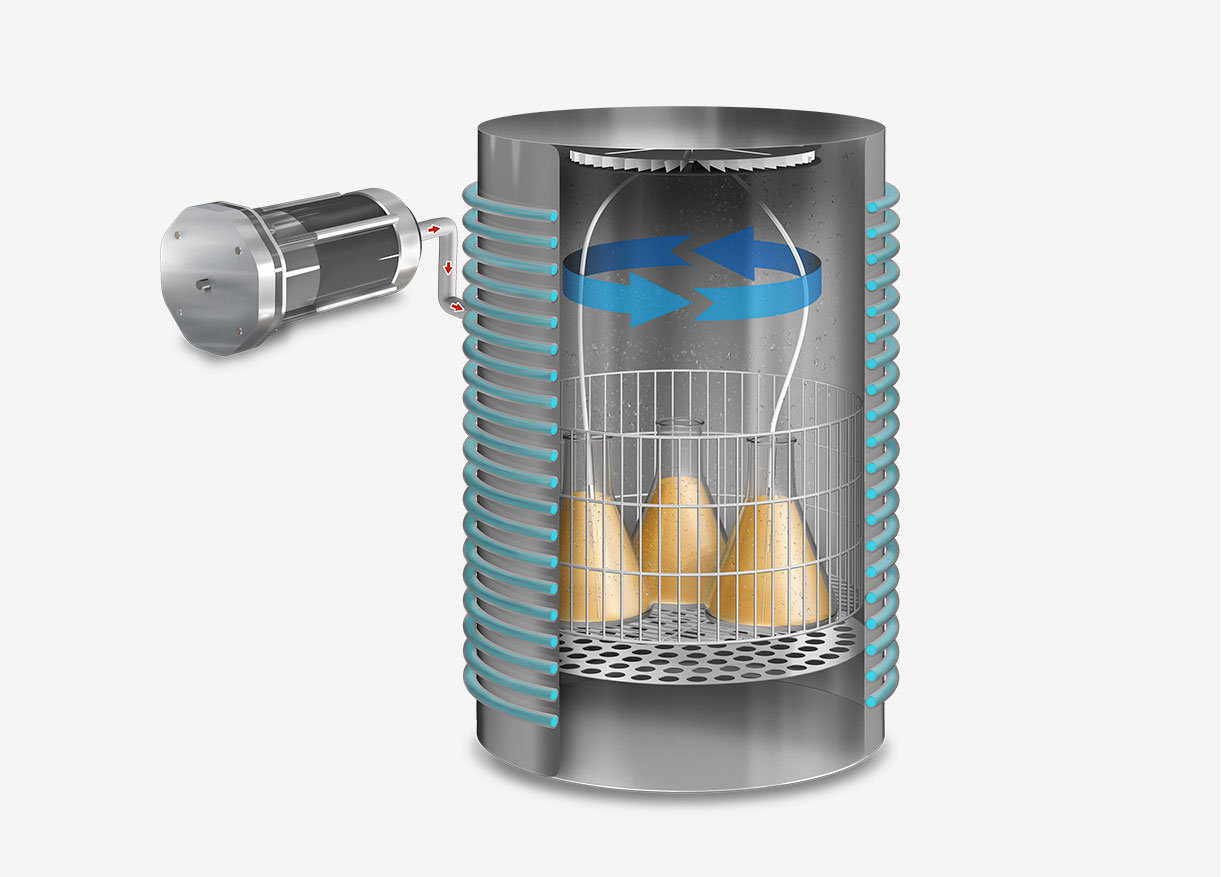
This sterilization cycle is characterized by its distinctive method of air removal from the chamber. Steam is generated at the chamber’s base, heating the air and reducing its density, thereby causing it to ascend and be expelled. Over a span of several minutes, the air fraction within the chamber is substantially diminished, leaving primarily saturated water vapor.
The process derives its name, “gravity cycle,” from the fundamental role of gravity in its operation. The method’s efficiency is attributed to its straightforwardness and the capability of pressurized steam to attain high temperatures, thereby facilitating effective heat transfer to the load. This makes it an ideal choice for cost-effective sterilization.
The gravity cycle is especially well-suited for materials with uncomplicated geometries that are non-moisture sensitive and capable of withstanding temperatures exceeding 121°C, such as glass, metals, and certain plastics. It is prevalently employed in university laboratories, industrial settings, research institutions, and healthcare environments to sterilize instruments lacking internal cavities, intricate geometries, or protective packaging.
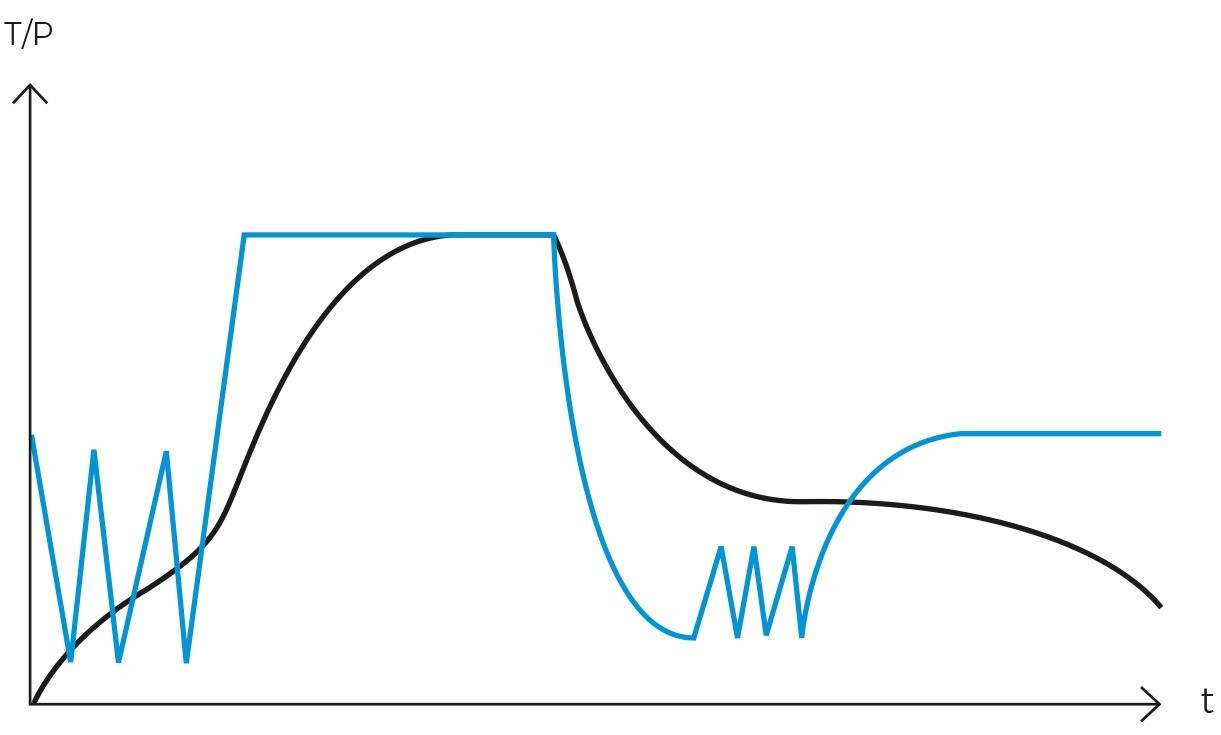
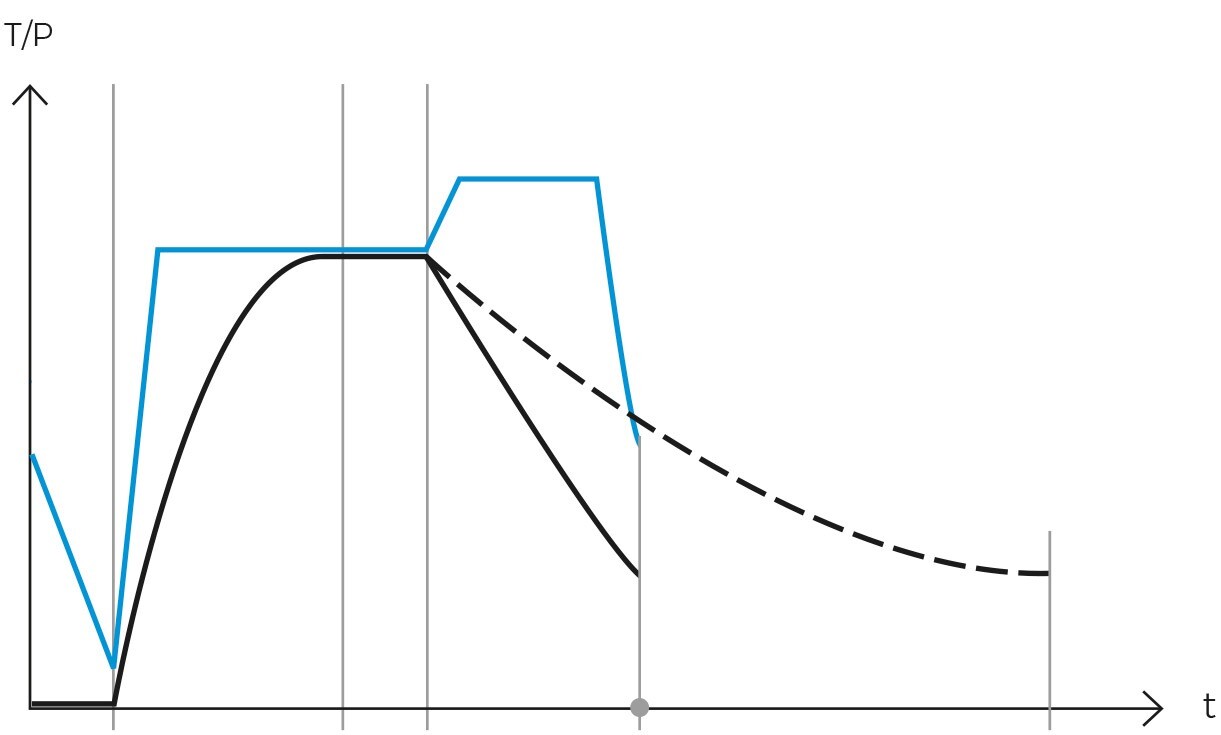
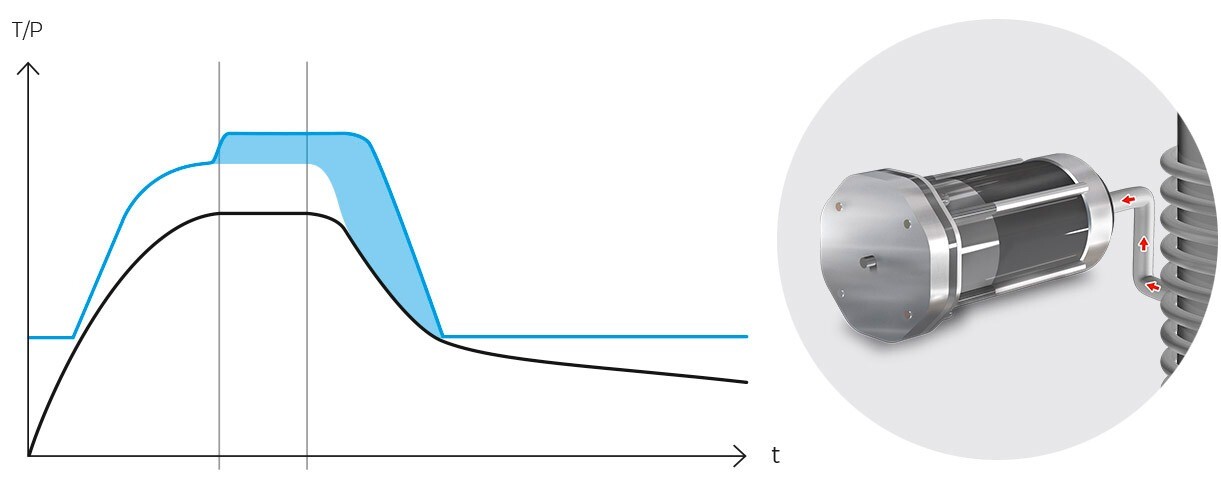
Operationally, the gravity cycle typically follows a specific sequence. Initially, the load is heated, and cold air is expelled. Subsequently, the purge valve is closed, leading to a rise in internal pressure until the sterilization temperature is achieved. Following this, the sterilization phase commences. Upon completion of the programmed exposure time, the natural cooling phase begins, during which the chamber and load progressively cool and depressurize.
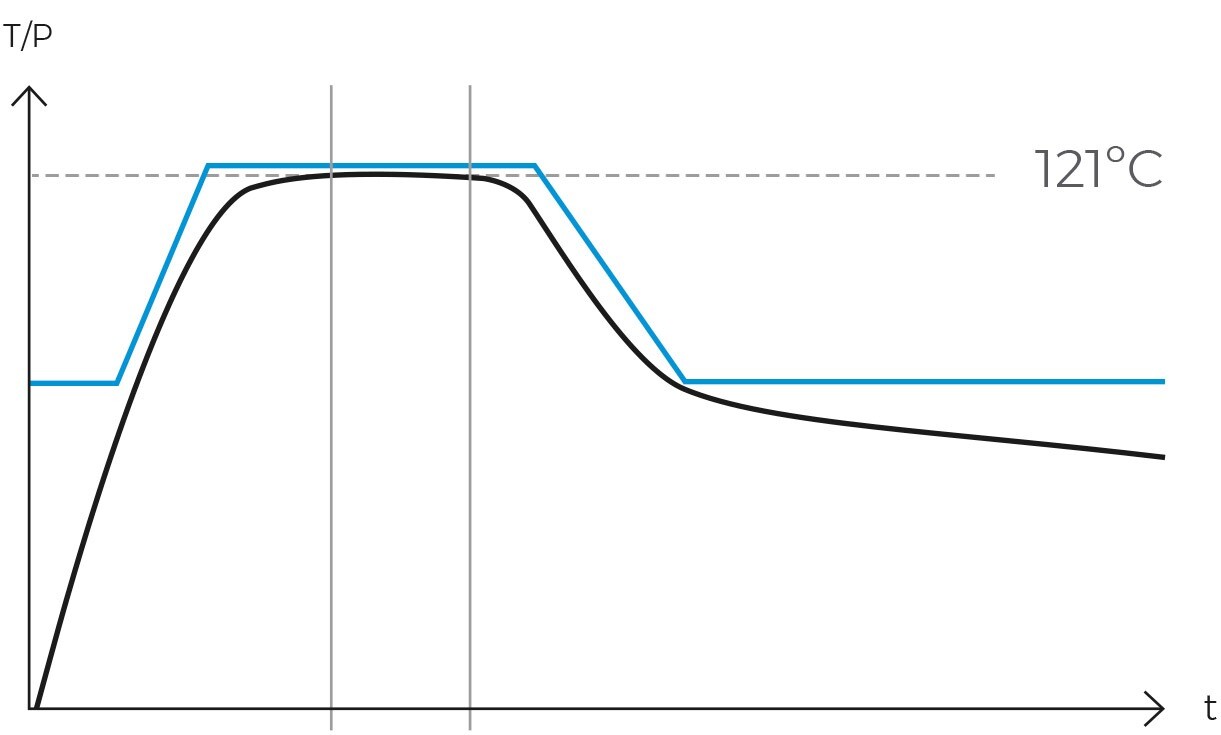
One of the principal advantages of the gravity cycle is its inherent simplicity. It obviates the need for vacuum systems, air compressors, heating jackets, steam generators, and additional pumps, thereby rendering autoclaves designed solely for these cycles significantly less expensive and remarkably easy to maintain. Moreover, the efficacy of this cycle in eradicating microorganisms has been extensively validated, establishing it as a dependable method for sterilizing a variety of objects.
Nevertheless, the gravity cycle presents certain limitations. It is unsuitable for the sterilization of porous materials or packaged items due to its suboptimal air elimination system. The residual air within the chamber acts as an insulator, impeding the steam from effectively permeating all surfaces of the load. Additionally, it is not appropriate for sterilizing hermetically sealed objects. In such instances, alternative sterilization methods, such as the vacuum cycle or the cycle with pressure support.
Principles of operation of the gravity cycle
The gravity cycle in an autoclave is renowned for its efficiency and simplicity, relying on the use of high-pressure, high-temperature steam. The key principles governing its operation are as follows:

-
Steam generation and use
The autoclave heats water to produce saturated steam, which is steam devoid of suspended water droplets. This steam permeates all areas of the sterilization chamber, ensuring efficient and uniform heat transfer. In basic autoclaves, steam is generated by heating elements located at the base of the autoclave. In more advanced models, an external steam generator injects saturated steam into the sterilization chamber.
-
Purge and air expulsion
Hot steam, being lighter than air, rises and displaces air out of the chamber through an open bleed valve during this step. This gravity-based process is critical for air removal, as the presence of cold air pockets would prevent the steam from contacting all surfaces of the items to be sterilized.
-
Sterilization phase
Once the air has been completely expelled, the chamber is sealed, and the steam temperature is raised to the sterilization temperature, typically 121ºC. The temperature and pressure are then maintained constant and without fluctuations for a specified period. This combination of temperature, pressure, and time ensures the complete transmission of energy from the steam to the load, effectively inactivating all microorganisms. The duration of this phase varies depending on the type of material being sterilized and the microbial load present.
-
Cooling and end of cycle
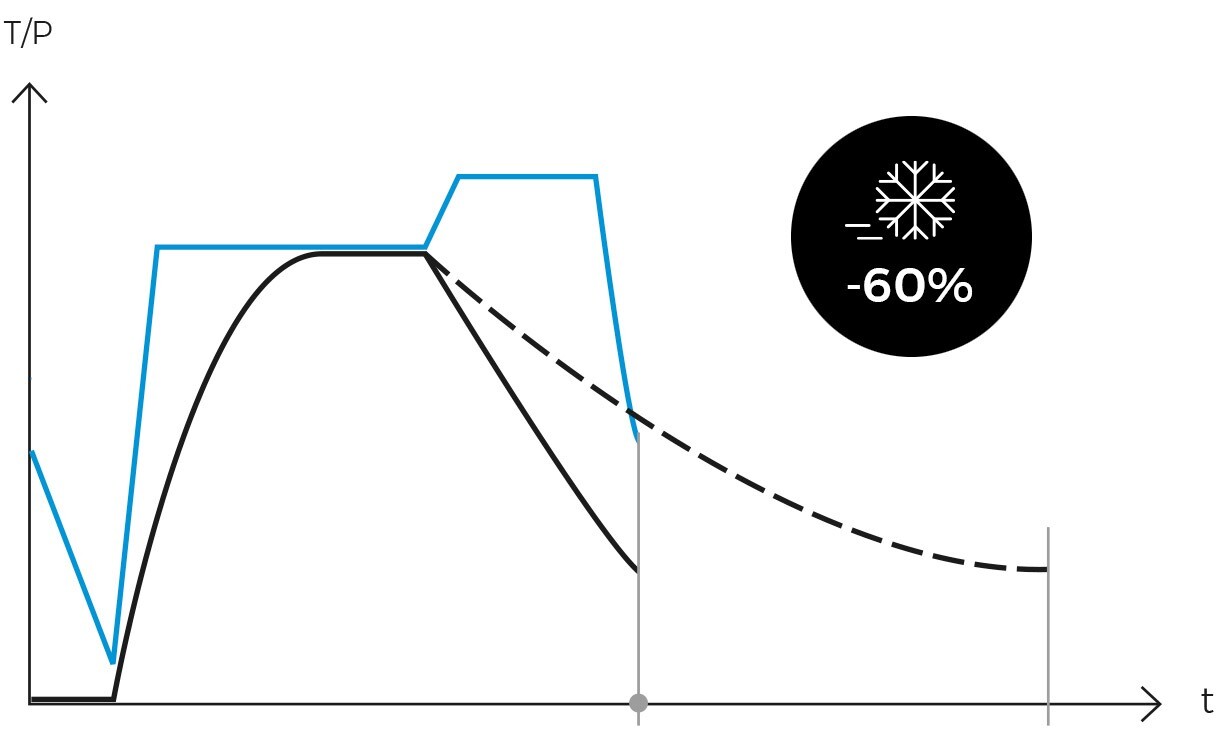
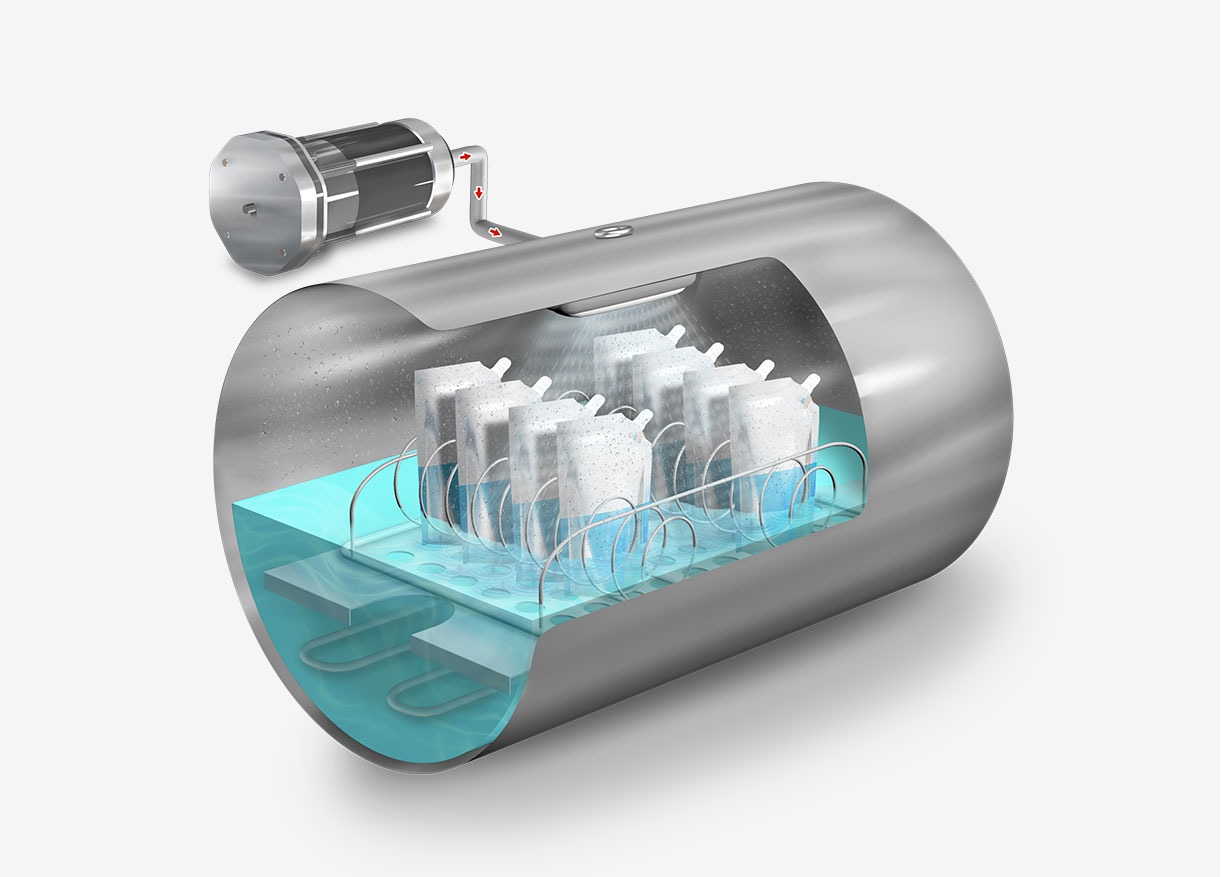
Following sterilization, the cooling phase begins. Steam is gradually released from the chamber, reducing pressure in a controlled manner and allowing items to cool. In programs designed for solid loads, this step is performed rapidly, while in liquid programs, it must be conducted more slowly and in stages to prevent splashing or breakage of containers due to sudden changes in temperature and pressure.
For solid loads, our AES Series autoclaves incorporate a push-button feature that allows the rapid release of steam from the chamber once the sterilization cycle is complete. In contrast, our more advanced models automatically perform this procedure in solid programs and may include drying systems to ensure that sterilized materials are dry and ready for use or storage.

-
Control and monitoring
Throughout the cycle, precise control of time, temperature, and pressure parameters is essential. Our autoclaves are equipped with automatic monitoring and control systems that ensure process accuracy and reproducibility. Additionally, they can be fitted with ticket printers and management software, enabling comprehensive control of all processes conducted within the autoclave.
Gravity cycle vs. vacuum cycle
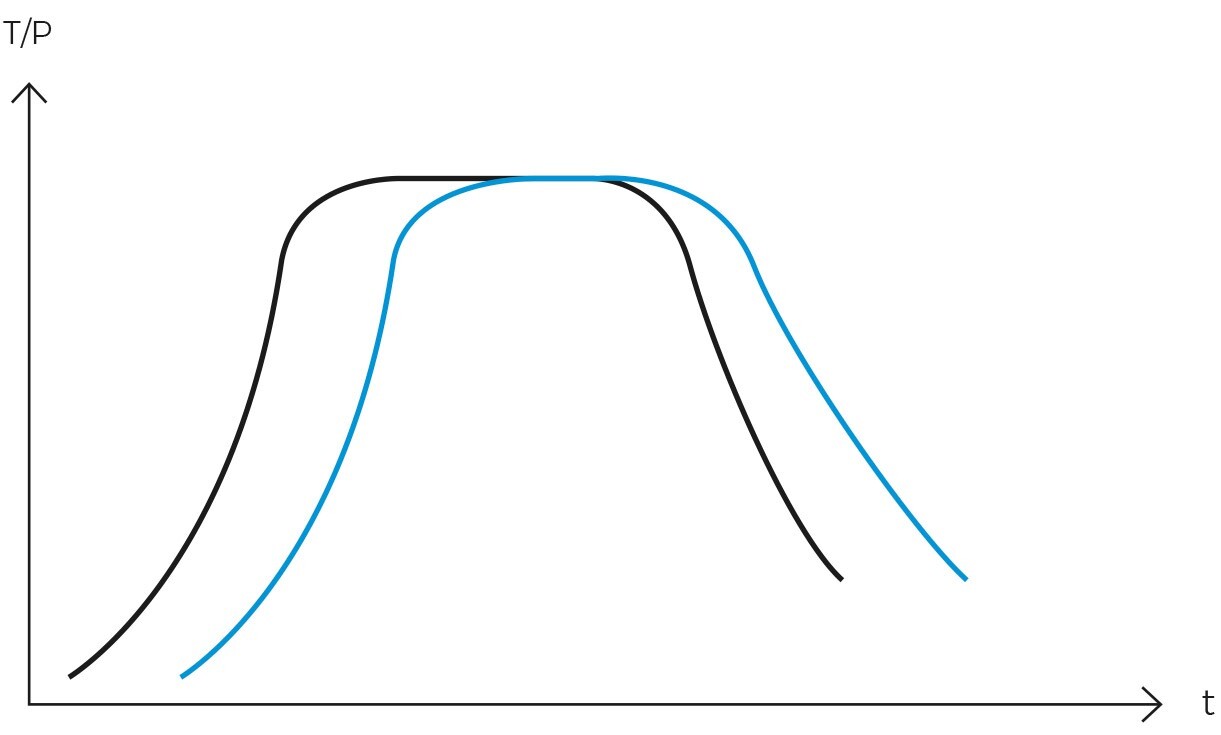
The selection between a gravity cycle and a vacuum cycle in autoclaves hinges on the specific sterilization needs of the laboratory. While a vacuum autoclave can perform a gravity cycle, an inexpensive autoclave designed solely for gravity cycles cannot execute a vacuum cycle. Therefore, it is imperative to choose the appropriate model at the time of purchase, considering both current and future sterilization requirements.
Gravity cycle
The gravity cycle is ideal for sterilizing solid objects, instruments, liquids, non-porous materials, and unbagged loads. It is widely used in laboratories, universities, schools, and research centers to sterilize glassware and simple instruments lacking internal cavities or complex geometries. Its primary advantage lies in its lower acquisition, maintenance, and operational costs, attributable to its straightforward design.
Vacuum cycle
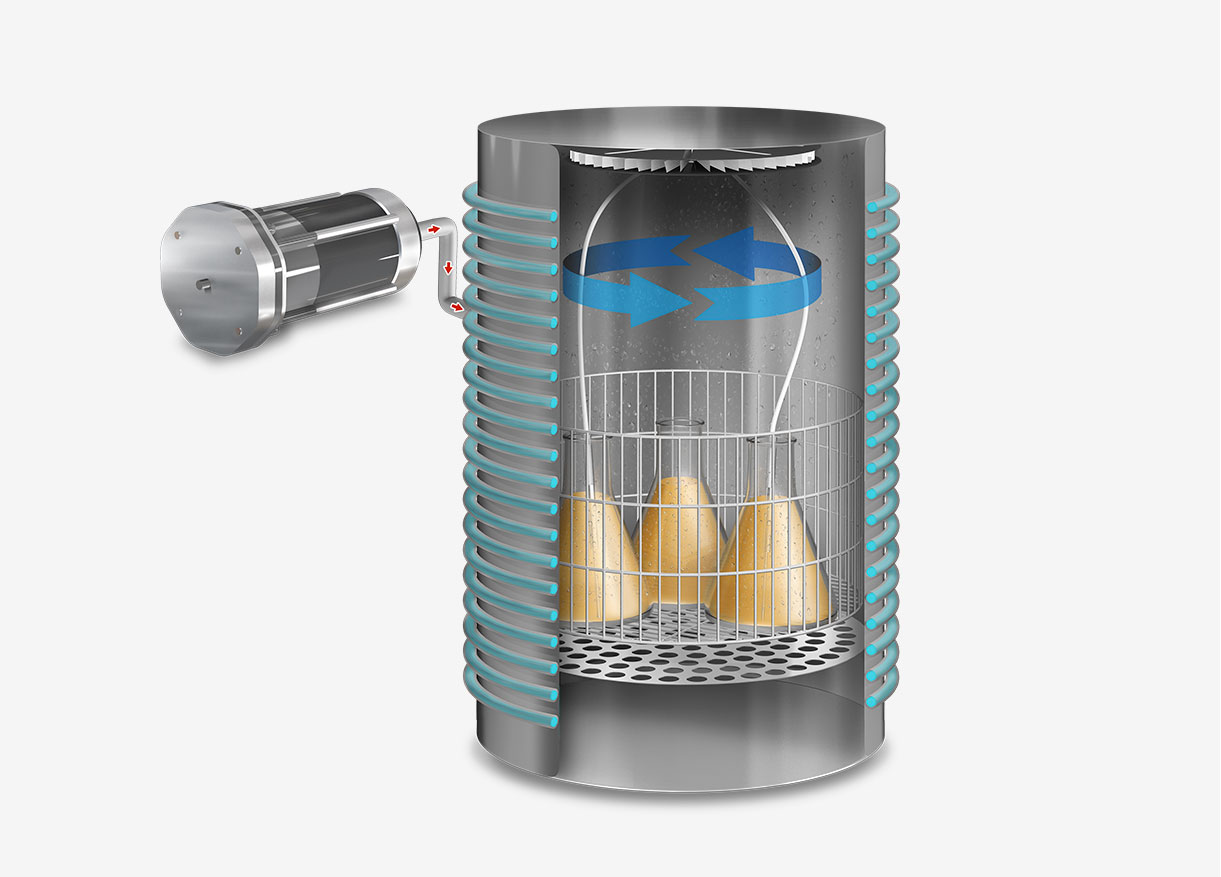
Conversely, the vacuum cycle involves creating a vacuum within the chamber before steam introduction. This process effectively eliminates all air pockets present in porous materials or objects with internal cavities, rendering it suitable for sterilizing more complex materials such as waste bags, surgical instruments with lumens, textiles, bagged objects, and bioreactors. Its chief advantage is the ability to provide effective sterilization for more challenging loads.
Which cycle is more efficient?
In terms of efficiency, the vacuum cycle surpasses the gravity cycle in sterilizing objects with hard-to-penetrate surfaces or porous materials. However, the gravity cycle is faster, more cost-effective, and sufficiently efficient for simple, non-porous loads.
From a cost and maintenance perspective, autoclaves operating with vacuum cycles are more expensive and necessitate more frequent and rigorous maintenance due to their components. These components may include a vacuum system, air compressor, heating jacket, bacteriological filter, fast cooling system, and steam generator. Additionally, they require a water purification system for operation.
Use cases of the gravity cycle
The gravity autoclave cycle, known for its popularity and simplicity, is used in a wide variety of industries. Here are some common applications of this cycle:
-
Research and life sciences laboratories
In research and life sciences laboratories, the gravity cycle is utilized for sterilizing culture media, aqueous solutions, metal instruments, and laboratory glassware. It is particularly effective for the routine sterilization of pipettes, flasks, and other glass containers.

-
Quality control laboratories in the food and beverage industry
In the food and beverage industry, quality control laboratories frequently use the gravity cycle to sterilize instruments, glassware, culture media, laboratory waste, and containers. Ensuring proper load sterilization is crucial to prevent contamination, thereby maintaining hygiene and quality standards throughout the industry.
-
Wastewater treatment plants
Quality control laboratories within wastewater treatment plants rely on the gravity cycle to manage microbiological contamination in wastewater and treated water. This cycle is used to sterilize laboratory glassware, laboratory residues, culture media, and solutions. It is particularly effective for the routine sterilization of test tubes, Petri dishes, funnels, burettes, and bottles containing liquids
-
Educational centers and universities
Autoclaves in educational centers and universities are often equipped exclusively with gravity cycle functionality due to its cost-efficiency and reliability. These devices are popular for effectively sterilizing aqueous liquid loads and culture media, as well as a wide variety of solid materials, including flasks, pipette tips, glass bottles, and Petri dishes.
-
Pharmaceutical, biotechnology and cosmetics industry
While the pharmaceutical, biotechnology, and cosmetics industries often prefer vacuum and pressure-supported cycles for the production of packaged products, the gravity cycle is still used for sterilizing liquids and glassware.
-
Clinical settings
In hospital laboratories, clinics, and healthcare facilities, the vacuum cycle is the most popular option. However, the gravity cycle is commonly used to process simple, unbagged items. It is ideal for sterilizing glassware or unpackaged metal instruments such as trays, scissors, tweezers, and other utensils. For more delicate items like laparoscopic instruments or medical implants, vacuum cycles are used instead.
-
Veterinary and animal care
Similar to clinical settings, veterinary hospitals and animal care centers use the gravity cycle to sterilize simple, unbagged loads. This practice minimizes the risk of disease transmission between animals and between animals and humans.
Critical factors to ensure gravity cycle efficiency
To guarantee the effectiveness of the gravity cycle in autoclave sterilization, several critical factors must be carefully controlled and monitored. These factors are essential for ensuring a safe and effective sterilization process:
-
Loading and distribution of materials
The manner in which materials are loaded into the autoclave significantly impacts the cycle’s efficiency. It is imperative to distribute objects evenly and avoid overpacking or stacking them too tightly, which could impede proper steam circulation.
-
Correct air elimination
The efficiency of the gravity cycle relies heavily on the complete removal of air from the sterilization chamber. Residual air can prevent saturated steam from reaching all surfaces of the objects, leading to incomplete sterilization. Therefore, it is essential to ensure that the purge process is executed correctly and that the purge valve is unobstructed.
-
Rigorous control of sterilization parameters
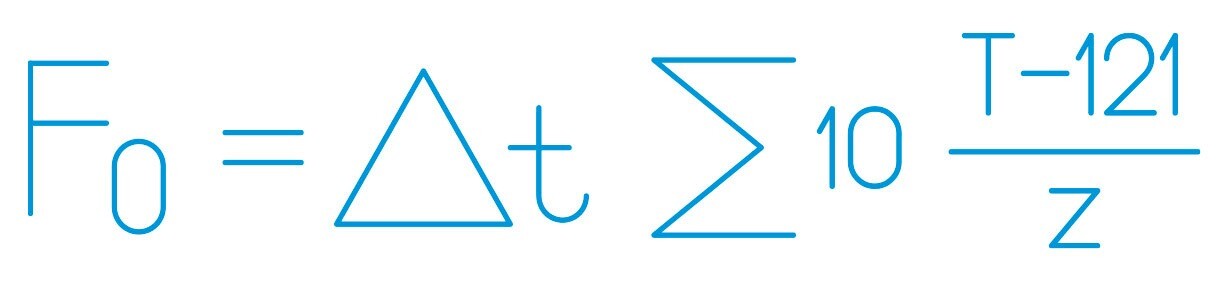
Maintaining proper temperature and pressure throughout the sterilization cycle is vital. Typically, achieving and sustaining a temperature of at least 121°C at a specific pressure is necessary to ensure the effective elimination of microorganisms. Each cycle’s results should be recorded and verified, supplemented by biological and/or chemical control elements such as sterilization control tape or spore tests, to validate the correct sterilization of the load.
-
Adequate exposure time
The duration of exposure to saturated steam must be sufficient to ensure thorough sterilization. This exposure time varies depending on the type of material being sterilized and the microbial load present. It is advisable to consult the manufacturer’s guidelines to ensure that the program parameters are appropriate. As a precaution, when in doubt, it is better to extend the sterilization time.
-
Autoclave maintenance and calibration
Regular maintenance and accurate calibration of the autoclave are crucial for optimal performance. This involves periodic checks of critical components such as seals, valves, and sensors, as well as precise calibration of temperature and pressure controls. Over time, the accuracy and precision of temperature probes that govern the autoclave’s sterilization cycle may degrade, necessitating periodic verification to ensure they are functioning correctly.
Safety and maintenance considerations
Ensuring the safe operation and proper maintenance of gravity cycle autoclaves is critical to preventing risks and maintaining the efficiency of the sterilization process. The following key considerations are essential in these areas:
Operator safety
The operation of autoclaves must adhere strictly to the manufacturer’s instructions and prevailing safety regulations. Operators must receive comprehensive training on the use of the equipment and be cognizant of the hazards associated with machinery that operates under high pressure and temperature conditions. It is critical to avoid opening the autoclave during its operational cycle to prevent the sudden release of hot, pressurized steam. Despite modern autoclaves being equipped with door locking systems during operation, it is important to note that the load may still be very hot, particularly bulky liquid loads, even after the cycle has concluded and the door is opened.
Preventive maintenance
A robust preventive maintenance program is essential for the optimal and safe operation of the autoclave. This program should encompass regular inspections of safety-critical components, including seals, valves, filters, and sensors, as well as the prompt replacement of any worn parts.
Calibration and periodic checks
Regular calibration of the temperature and pressure controls is crucial to ensure the autoclave’s proper functioning. Additionally, periodic checks using sterilization control tape or spore tests are recommended to verify the effectiveness of each sterilization cycle.
Use of clean water
It is imperative to periodically renew the water used in the autoclave and to employ only distilled or purified water. This practice prevents the transfer of salts to the load and the formation of salt deposits on the walls and inner surfaces of the sterilization chamber over time.
Cleaning and disinfection
Routine cleaning and disinfection of the sterilization chamber and other autoclave components are vital to prevent residue accumulation, salt deposit formation, and cross-contamination. The manufacturer’s guidelines for appropriate cleaning and disinfecting agents should be meticulously followed.

Handling of sterilized materials
Post-sterilization, materials must be handled with care to maintain their sterility. This includes allowing items to cool adequately before handling and storing them in a clean, dry environment. In the absence of a drying autoclave, a laboratory oven should be used to dry solid loads before use.
Registration and documentation
Maintaining detailed records of sterilization cycles, maintenance activities, and any incidents is crucial for quality control. These records are also essential for compliance with regulations and quality standards such as Good Laboratory Practice (GLP).